Harold A. Fisk
Associate Professor
216 Biological Sciences Building
484 West 12th Avenue
Columbus, OH
43210-1292
Areas of Expertise
- Cell Biology
- Centrosome Duplication
- Cell Cycle Regulation
- Mitotic Spindle
Education
- B.A. 1989, University of Colorado, Boulder.
- Ph. D. 1998, University of California, San Diego.
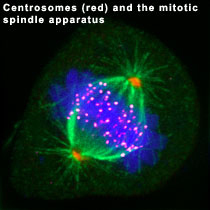
My laboratory is interested in understanding how the centrosome organelle is assembled, and how defects in this process might lead to the chromosome segregation errors associated with human cancer cells. Centrosomes are microtubule-organizing centers that act as poles of the mitotic spindle apparatus (shown at the right) to regulate its assembly and function. Centrosomes make additional contributions to chromosome segregation by regulating cytokinesis and the decision to enter the subsequent cell cycle. It is therefore easy to imagine how defects in centrosome function might cause chromosome segregation errors. Because centrosomes make many contributions to the fidelity of chromosome segregation, and because most human tumors are aneuploid as a result of errors in chromosome segregation, it is not surprising that defects in the number, structure, and function of centrosomes are associated with many human tumors.
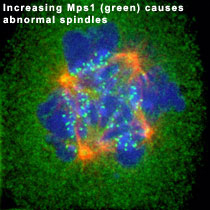 Like the genome, centrosomes must be precisely duplicated prior to cell division. We have shown that the Mps1 protein kinase is present at centrosomes and its kinase activity is required for their duplication. The level of Mps1 at centrosomes must be tightly controlled, because increasing the dosage of Mps1 at centrosomes causes the production of extra centrosomes, leading to the assembly of abnormal mitotic spindles like that shown at the left. Mps1 is normally an unstable protein, and its accumulation at centrosomes is controlled by proteasome-mediated degradation (as diagramed below).
Like the genome, centrosomes must be precisely duplicated prior to cell division. We have shown that the Mps1 protein kinase is present at centrosomes and its kinase activity is required for their duplication. The level of Mps1 at centrosomes must be tightly controlled, because increasing the dosage of Mps1 at centrosomes causes the production of extra centrosomes, leading to the assembly of abnormal mitotic spindles like that shown at the left. Mps1 is normally an unstable protein, and its accumulation at centrosomes is controlled by proteasome-mediated degradation (as diagramed below).
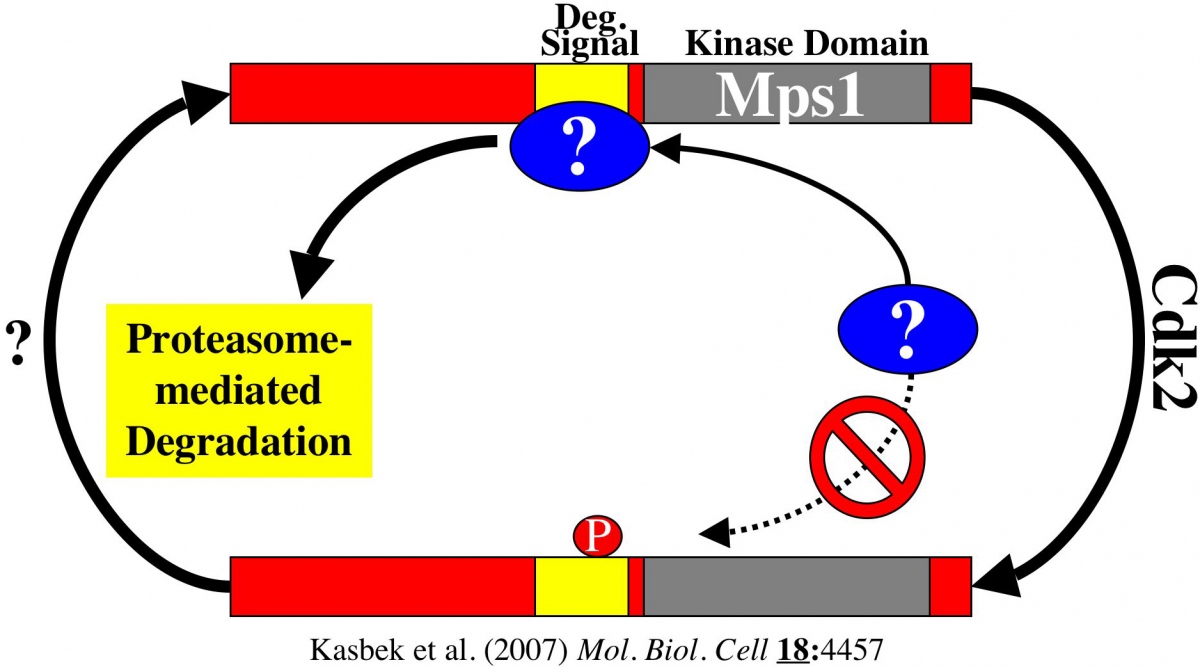
Cdk2 activity serves to transiently suppress this degradation, restricting centrosome duplication to a limited window in the cell cycle when Cdk2 is present. Specifically, cyclin A- associated Cdk2 complexes phosphorylate the Mps1 degradation signal, and this phosphorylation protects Mps1 from degradation. A mutant version of Mps1 that cannot be phosphorylated by Cdk2 cannot substitute for the the function of the endogenous Mps1 protein because it is always degraded at centrosomes. In contrast, either removing the Mps1 degradation signal or mimicking constitutive phosphorylation by Cdk2 prevents Mps1 degradation and causes the production of extra centrosomes.
Interestingly, Mps1 is aberrantly stable in many human tumor-derived cells. We have found that one such cell line expresses a mutant form of Mps1 that lacks the degradation signal. This non-degradable mutant protein deregulates centrosome duplication in a variety of cell types. We have also found that the machinery that targets unphosphorylted Mps1 to the proteasome is defective in a second tumor derived cell line.
The goal of our research is to understand the mechanisms of centrosome duplication in human cells, and to determine how this process is regulated and integrated into the cell cycle. We are using a combination of cell biological, biochemical, and molecular biological approaches to investigate the regulation and function of Mps1, and explore the relationships between Mps1 stability,centrosome duplication, and tumorigenesis. Ongoing projects in the lab include:
-
characterization of Mps1 targeting and binding partners
-
identification of factors that regulate function of the Mps1 degradation signal
-
identification of factors that target Mps1 for degradation
-
identification and characterization of Mps1 substrates
-
characterization of the role of Mps1 in centrosome defects and tumorigenesis
Dr. Fisk has a joint appointment in the Human Cancer Genetics Program, Department of Molecular Virology, Immunology, and Medical Genetics, OSU College of Medicine: http://cancergenetics.med.ohio-state.edu/3237.cfm
FISK LAB MEMBERS:
Graduate students:
Dwitiya Sawant (MG)
Joey Marquardt (MG)
Tan Nguyen (MG)
Jennifer Perkins (MCDB)
Undergraduate Students:
Tyler Brown (Biochemistry)
Research Assistant:
Ian Landis
Postdoctoral Fellow:
Shubhra Majumder, Ph.D.
Dwitiya Sawant, Ph.D.
Publications
-
Sawant, D. B., Majumder, S., Perkins, J. L., Yang, C.-H., Eyers, P. A., and Fisk, H. A. (2015) Centrin 3 is an inhibitor of centrosomal Mps1 and antagonizes Centrin 2 function. Mol. Biol. Cell in press.
-
Majumder, S., Cash, A., and Fisk, H. A. (2015) Non-Overlapping Distributions and Functions of the VDAC Family in Ciliogenesis. Cells 4:331.
-
Majumder S., and Fisk., H. A. (2014) Quantitative Immunofluorescene Assay to Measure the Variation in Protein Levels at Centrosomes. J. Vis. Exp. 94:e52030
-
Majumder, S., and Fisk, H.A. (2013) VDAC3 and Mps1 negatively regulate ciliogenesis. Cell Cycle 12(5):849.
-
Majumder S., Slabodnick M., Pike A., Marquardt J., Fisk, H.A. (2012) VDAC3 regulates centriole assembly by targeting Mps1 to centrosomes. Cell Cycle 11(19):3666.
-
Mishra, A., Liu, S., Sams, G. H., Curphey, D. P., Santhanam, R., Rush, L. J., Schaefer, D., Falkenberg, L. G., Sullivan, L., Jaroncyk, L. Zou, X., Fisk, H., Labanowska, J., Caserta, E., Wu, L.-C., Becker, H., Chandler, J. C., Wu, Y.-Z., Heerema, N. A., Chan, K. K., Zhang, J., Porcu, P., Racke, F. K., Garzon, R., Lee, R. J., Marcucci G., and Caligiuri, M. A. (2012) Aberrant Overexpression of IL-15 Initiates Large Granular Lymphocyte Leukemia through Chromosomal Instability and DNA Hypermethylation. Cancer Cell 22(5):645.
-
Fisk, H.A., “Many Pathways to Destruction: The Role of the Centrosome in, and Its Control by Regulated Proteloysis," in H. Schatten, ed., The Centrosome (Humana Press), 2012,133-156.
-
Liu J., Cheng X., Zhang Y., Li S., Cui H., Zhang L., Shi R., Zhao Z., He C., Wang C., Zhao H., Zhang C., Fisk, H.A., Guadagno T. M., and Cui Y. (2012)Phosphorylation of Mps1 by BRAF(V600E) prevents Mps1 degradation and contributes to chromosomeinstability in melanoma. ePub Mar 19 2012.
-
Liu, C., van Dyk, D., Choe, V., Yan, J., Majumder, S., Costanzo, M., Bao, X., Boone, C., Huo, K., Winey, M., Fisk, H.A., Andrews, B., and Rao, H. (2011) Ubiquitin Ligase Ufd2 Is Required For Efficient Degradation Of Mps1 Kinase. J. Biol. Chem. ePub Nov. 1, 2011.
-
Pike, A. N. and Fisk, H.A. Centriole assembly and the role of Mps1: defensible or dispensable? Cell Division (2011) 14(6):9.
-
Fisk, H.A., The Mip-ing link: Mip1 links Mps1 to the actin cytoskeleton. Cell Cycle (2011), 10:783.
-
Yang. C.-H., C. Kasbek, S. Majumder, A. Mohd Yusof, and H. A. Fisk, Mps1 Phosphorylation Sites Regulate the Function of Centrin 2 in Centriole Assembly. Mol Biol Cell (2010), 21:4361. ePub Oct. 27.
-
Kasbek, C., C.-H. Yang, and H. A. Fisk, Antizyme Restrains Centrosome Amplification by Regulating the Accumulation of Mps1 at Centrosomes. Mol Biol Cell (2010), 21:3878. ePub Sep. 22.
-
Kasbek, C., C.-H. Yang, and H. A. Fisk, Mps1 as a link between centrosomes and genomic instability. Env Mol Mutagenesis (2009) 50:654
-
Kasbek, C., C.-H. Yang, and H. A. Fisk, The use of infrared fluorescent dyes in immunofluorescence microscopy. in The Protein Protocols Handbook, 3rd Ed. (2009).
-
Yang, C.-H., C. Kasbek, and H. A. Fisk, The use of infrared fluorescent dyes in quantitative immunoblotting. in The Protein Protocols Handbook, 3rd Ed. (2009).
-
Kasbek, C. C.-H. Yang, A. Mohd Yusof, H. M. Chapman, M. Winey, and H. A. Fisk, Preventing the degradation of Mps1 at centrosomes is sufficient to cause centrosome re-duplication in human cells. Mol Biol Cell (2007) 18:4457-69.
-
Fisk, H.A. and M. Winey, Spindle Regulation: Mps1 flies into new areas. Current Biology (2004) 14:R1058-60.
-
Fisk, H.A., C.P. Mattison, and M. Winey, A field guide to the Mps1 family of protein kinases. Cell Cycle (2004) 3:439-442.
-
Fisk, H.A., C.P. Mattison, and M. Winey, Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc Natl Acad Sci U S A (2003) 100:14875-80.
-
Fisk, H.A., C.P. Mattison, and M. Winey, Centrosomes and tumour suppressors. Curr Opin Cell Biol (2002) 14:700-5.
-
Fisk, H.A. and M. Winey, The mouse mps1p-like kinase regulates centrosome duplication. Cell (2001) 106:95-104.
