Hay-Oak Park

Contact Information
Professor
Areas of Expertise
- Cell Signaling for Growth and Division
- Cellular Aging and Lifespan Control
Research Interests
Our work aims to discover conserved mechanisms underlying the control of cell polarization and cellular lifespan in eukaryotes. Cell polarity is a universal feature of living cells from bacteria to humans. Polarity establishment is critical for cell proliferation and development. Abnormal regulation of cell polarity and asymmetry has been implicated in disease processes, such as cancer, and cellular aging. Cell growth and divisions are tightly controlled and coordinated with each other and impact life cycle and lifespan. Our current research has two main focuses: 1) spatiotemporal regulation of cell polarity establishment and organization of septins, a conserved family of GTPases that form filaments and high-order ultra structures; and 2) control of cellular aging and lifespan.
How do cells grow and divide at a specific site and at the right time?
In yeast and animal cells, signaling pathways involving small molecular weight GTPases regulate cell polarization. Cell growth and division of budding yeast are spatially and temporally controlled. We discovered polarization of the Cdc42 GTPase, an evolutionarily conserved regulator of cell polarity, involves two waves of activation in correlation with temporal steps of the G1 phase of the cell cycle. We further discovered the functional significance of biphasic activation of Cdc42 in coordinating multiple events for polarized growth, such as proper assembly of the septin ring and targeted secretion.
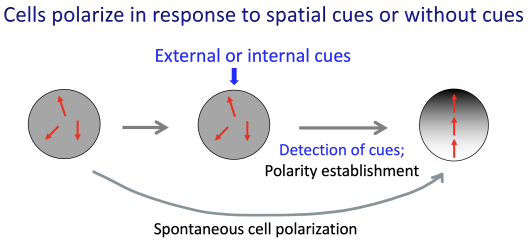
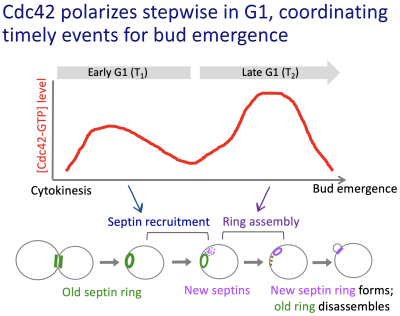
How is cellular lifespan determined?
Asymmetric cell division produces a mother cell with a limited lifespan and a daughter cell with full lifespan potential in yeast, analogous to a stem cell lineage in animals. Given that cell polarity is essential for asymmetric cell division, we asked how polarity establishment is disrupted in aging cells and what causes cell proliferation to stop. Our work uncovered the upregulation of Cdc42 during aging and suggested a negative correlation between Cdc42 activity and lifespan. Increased Cdc42 activity has also been associated with a depolarized phenotype, symmetric cell divisions, and aging in stem cells. We investigate the mechanisms underlying hyperactivation of Cdc42 during aging as well as the signaling outputs of the network that limit lifespan.

SELECTED PUBLICATIONS since 2007 (*Corresponding Author):
- Kang, P. J., R. Mullner, K. Lian, H.-O. Park* (2023). Cdc42 couples septin recruitment to the axial landmark assembly via Axl2 in budding yeast. J Cell Sci. 2023 Sep 15;. doi: 10.1242/jcs.261080. [Epub ahead of print] PMID: 37712304.
- Liu, Y., J. Xie, H.-O. Park, W.-C. Lo* (2023). Mathematical modeling of cell polarity establishment of budding Yeast. Commun. Appl. Math. Comput.. 2023 February. doi: https://doi.org/10.1007/s42967-022-00240-y.
- Kang, P. J., R. Mullner, H. Li, D. Hansford, H. W. Shen, and H.-O. Park* (2022) Upregulation of the Cdc42 GTPase limits the replicative lifespan of budding yeast. Mol Biol Cell. 2022 Jan 19: Online ahead of print. PMID: 35044837
- Miller, K. E., P. J. Kang, and H.-O. Park* (2020) Regulation of Cdc42 for polarized growth in budding yeast. Microb Cell. 2020 May 19;7(7):175-189. PMID: 32656257. https://pubmed.ncbi.nlm.nih.gov/32656257/
- Miller, K. E., W.-C. Lo, C.-S. Chou, and H.-O. Park* “Temporal regulation of cell polarity via the interaction of the Ras GTPase Rsr1 and the scaffold protein Bem1”. Mol Biol Cell. (2019) Epub 2019 Aug 14.
- Singh, K., M.E. Lee, M. Entezari, C.-H. Jung, Y. Kim, Y. Park, J. D. Fioretti, W.-K. Huh*, H.-O. Park* and P. J. Kang* (2019) Genome-Wide Studies of Rho5-Interacting Proteins That Are Involved in Oxidant-Induced Cell Death in Budding Yeast. G3 (Genes/Genomes/Genetics), Epub 2019 Jan 22.
- Kang, P. J., K. E. Miller, J. Guegueniat, L. Beven, and H.-O. Park* (2018) The shared role of the Rsr1 GTPase and Gic1/Gic2 in Cdc42 polarization. Mol Biol Cell. 2018 Oct 1;29(20):2359-2369. doi: 10.1091/mbc.E18-02-0145. Epub 2018 Aug 9.
- Carmona-Gutiérrez, D.* et al., …… H.-O. Park, ….…, F. Madeo* (2018) “Guidelines and recommendations on yeast cell death nomenclature” Microb Cell. 2018 Jan 1; 5(1): 4–31.
- Miller, K. E., W.-C. Lo, M.E. Lee, P. J. Kang, and H.-O. Park* (2017) “Fine-tuning the orientation of the polarity axis by Rga1, a Cdc42 GTPase activating protein”,Mol Biol Cell. 2017 Dec 15;28(26):3773-3788. Epub 2017 Oct 26.
- Okada, S., M. E. Lee, E. Bi E, H.-O. Park* (2017) Probing Cdc42 Polarization Dynamics in Budding Yeast Using a Biosensor. Methods Enzymol. 2017;589:171-190. Epub 2017 Feb 20.
- Lee, M. E.@, W.-C Lo@, K. E. Miller, C.-S. Chou, and H.-O. Park* (2015) Regulation of Cdc42 polarization by the Rsr1 GTPase and Rga1, a Cdc42 GTPase activating protein, in budding yeast. J Cell Sci 128: 2106-2117 (@ equal contribution)
Highlighted in “In This Issue” of the J Cell Sci 2015 128:e1103: - Miller, K. E., Y. Kim, W.-K. Huh, and H.-O. Park* (2015) Bimolecular Fluorescence Complementation (BiFC Analysis): advances and recent applications for genome-wide interaction studies J Mol Biol [2015 Mar 12; Epub ahead of print] — invited review
- Lam, M. H. Y., J. Snider1, M. Rehal, V. Wong, F. Aboualizadeh, L. Drecun, O. Wong, B. Jubran, M. Li, M. Ali, M. Jessulat; V. Dieneko, R. Miller, M. E. Lee, H.-O. Park, A. Davidson, M. Babu, and I. Stagljar* (2015) A comprehensive membrane interactome mapping of Sho1p reveals Fps1p as a novel key player in the regulation of the HOG Pathway in S. cerevisiae. J Mol Biol [2015 Jan 30; Epub ahead of print]
- Kang, P.J., M.E. Lee, and H.-O. Park* (2014) Bud3 activates Cdc42 to establish a proper growth site in budding yeast. J Cell Biol 206: 19 - 28. Highlighted in “In This Issue” of the J Cell Biol.
- Lo, W.-C.*, H.-O. Park, and C.-S. Chou (2014) Mathematical Analysis of Spontaneous Emergence of Cell Polarity Bull. Math. Biol. 76(8): 1835-65.
- Snider, J., A. Hanif, M. E. Lee, K. Jin, A. R Yu, C. Graham, M. Chuk, D. Damjanovic, M. Wierzbicka, P. Tang, D. Balderes, V. Wong, M. Jessulat, K. D. Darowski, B.-J. San Luis, I. Shevelev, S. L. Sturley, C. Boone, J. F. Greenblatt, Z. Zhang, C. M. Paumi, M. Babu, H.-O. Park, S. Michaelis, and I. Stagljar* (2013) Mapping the functional yeast ABC transporter interactome. Nature Chem Biol. 9(9):565-72 [Epub 2013 Jul 7].
- Lo, W.-C. #,M. Lee#,M. Narayan,C.-S. Chou,and H.-O. Park* (2013) Polarization of diploid daughter cells directed by spatial cues and GTP hydrolysis of Cdc42 in budding yeast. PLoS ONE 8(2):e56665. [Epub 2013 Feb 20]; #equal contribution to the work.
- Kang, P. J., J. K. Hood-DeGrenier, and H.-O. Park* (2013) Coupling of septins to the axial landmark by Bud4 in budding yeast. J Cell Sci. 126:1218-26 [Epub 2013 Jan 23].
- Bi, E. and H.-O. Park (2012) Cell polarization and cytokinesis in budding yeast. Genetics 191: 347-387.
- Nelson, S. A., A. M. Sanson, H.-O. Park, and J. A. Cooper* (2012) A novel role for the GTPase activating protein Bud2 in the spindle position checkpoint. PLoS ONE 7(4): e36127.
- Kang, P. J., E. Angerman, C.-H. Jung, and H.-O. Park* (2012) Bud4 mediates the cell-type-specific assembly of the axial landmark in budding yeast. J Cell Sci. 125:3840-9
- Lee ME, Singh K, Snider J, Shenoy A, Paumi CM, Stagljar I, Park HO* (2011) The Rho1 GTPase Acts Together With a Vacuolar Glutathione S-conjugate Transporter to Protect Yeast Cells from Oxidative Stress. Genetics. 188(4):859-70.
- Kang, P. J., L. Beven, S. Hariharan and H.-O. Park* (2010) The Rsr1/Bud1 GTPase interacts with itself and the Cdc42 GTPase during bud-site selection and polarity establishment in budding yeast. Mol. Biol. Cell 21: 3007-3016.
- Kozminski, K. G. and Park, H.-O.* (2009) Yeast small G protein function: Molecular basis of cell polarity in yeast, in Handbook of Cell Signaling, Second Ed., eds. R. A. Bradshaw & E. A. Dennis, Vol. 2, pp. 1813-1817, Academic Press, Elsevier Inc.
- Singh, K., P. J. Kang, and H.-O. Park* (2008) The Rho5 GTPase is necessary for oxidant-induced cell death in budding yeast. Proc. Natl. Acad. Sci. USA 105: 1522-1527
- Park, H.-O.* and E. Bi (2007) Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiology & Molecular Biology Reviews, 71: 48-96
